Chemical Bonding In Solids Burdett Pdf
- Chemical Bonding In Solids Burdett Pdf Answers
- Chemical Bonding In Solids Burdett Pdf Answer
- Chemical Bonding In Solids Burdett Pdf Printable
Chemistry 253

MaterialsChemistry
Spring 2016
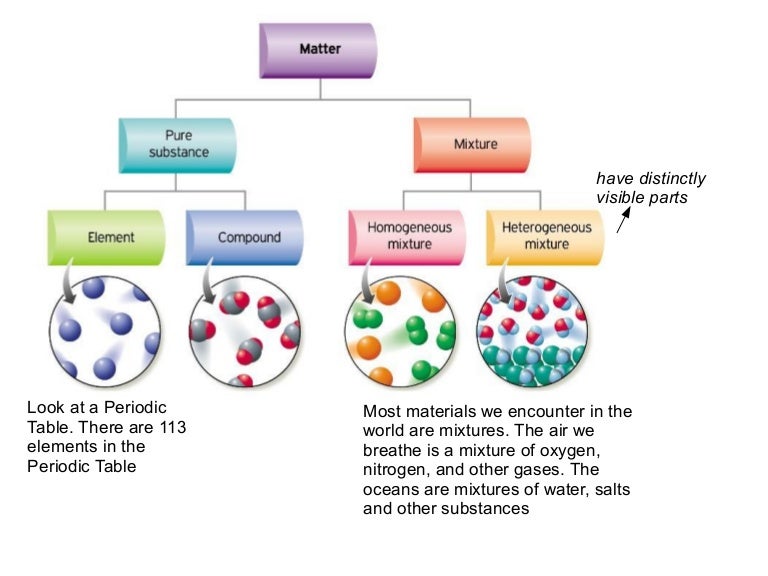
MATERIALS SCIENCE AND ENGINEERING – Vol. I – Bonding in Solids, Structural and Chemical Properties - R. Grimes ©Encyclopedia of Life Support Systems (EOLSS) BONDING IN SOLIDS, STRUCTURAL AND CHEMICAL PROPERTIES R. Grimes Imperial College, London, UK Keywords: Atomic structure, band structure, bonding, bulk modulus, conductivity. In Chemical Bonding in Solids, renowned chemist Jeremy K. Burdett offers a clear and much-needed synthesis of chemical bonding theory and solid state structural considerations. Over the past fifteen years, the delocalized orbital model favored by molecular chemists-the model of choice for understanding a plethora of organic, inorganic,. Bonding energy between the two atoms. To break the bond, this energy must be supplied from outside. Breaking the bond means that the two atoms become infinitely separated. In real materials, containing many atoms, bonding is studied by expressing the bonding energy of the entire materials in terms of the separation distances between. The electronic structure of solids, 89 4.1 Oxides with the NaCl, Ti02, and Mo02 Structures, 89 4.2 The Diamond and Zincblende Structures, 96 4.3 'Localization' of 'Delocalized' Orbitals in Solids, 102 4.4 The Structure of NbO, 109 4.5 Chemical Bonding in Ionic Compounds, 115 4.6 The Transition Metals, 117.
Instructor:
Professor Peidong Yang,
239 Hild.,Tel: 643-1545
Email: p_yang@berkeley.edu
Office Hours:Friday10-12pm, 239 Hild.
Nick Kornienko
nick.kornienko.berkeley@gmail.com
Description:
This course is intendedprimarily as an introduction course to materials and solid state chemistry forgraduate students and advanced undergraduate students. The objective is tounderstand solids from a chemical perspective and introduce general solid statesynthesis methodologies and characterization techniques. Topics covered willinclude: structure and structure determination of crystalline solids; freeelectron model for metals; electronic band structure; chemical bonding insolids; structure-property relationships.
Grading:
Problem sets (2)40%
Final60%
Main Reference Books: (Reservedat Chem./Phys./Engineering Lib.)
West: Solid State Chemistry and its Applications (Wiley,1988)
West: Basic solid state chemistry (Wiley, 1999).
Gersten and Smith: The Physics andChemistry of Materials (Wiley, 2001)
Hoffmann: Solids and Surfaces (VCH, 1988)
Burdett: Chemical Bonding in Solids (Oxford 1995)
Ashcroft and Mermin: Solid StatePhysics (Saunders College, latest edition)
Kittel: Introduction to solid State Physics (Wiley, 1996)
William D. Callister Jr. Materials Science and Engineering,an Introduction (Wiley)
Chemical Bonding In Solids Burdett Pdf Answers
Cheetham and Day: Solid StateChemistry: Techniques (Oxford, 1987)
Cheetham and Day: Solid StateChemistry: Compounds (Oxford, 1992)
Cox: The Electronic Structure and Chemistry of Solids(Oxford, 1987)
Wells: Structural Inorganic Chemistry (Clarendon Press,1984)
Wold and Dwight: Solid StateChemistry (Chapman Hall, 1993)
Courtesy ofDr. S. Heyes,Univ. Oxford, for crystal structural models in the Lectures.
Chemical Bonding In Solids Burdett Pdf Answer
Hereis a tentative list of the lecture topics.
Date
Materials Chemistry I
1 | ||
2 | Ashcroft: 4-7 | |
3 | Ashcroft: 4-7 | |
4 | Homework#01-Due March 10th | West: 5 |
5 | West: 7,8 | |
6 | Homework#02 – Due March 17th | |
7 | West: 11, 12 | |
8 | Callister 9 | |
9 | Phase Diagram | |
10 | Final |
Materials Chemistry II
11 | Ashcroft/Mermin: 1-3, 8-10 Kittel: 6-9 |
12 | Nearly free electron model, Fermi surface, |
13 | Fermi’s Golden Rule, Optical Transition |
14 | Gersten, 7,11 |
15 | Hoffmann,Burdett 1-3 |
16 | |
17 | |
18 | Tight Binding Model |
Chemical Bonding In Solids Burdett Pdf Printable
Materials Chemistry III
